

RNAI THERAPY OFFERS THE POTENTIAL TO REVOLUTIONIZE THE BIOPHARMACEUTICAL INDUSTRY
Owing to their vast potential in controlling disease-associated gene expression, RNAi therapeutics have emerged as a key segment of the market; several big pharma players have undertaken RNAi focused initiatives.
In addition to effective gene silencing, these candidates can be deployed for applications related to precision medicine. Studies have also demonstrated the safety of RNAi therapeutics for in systemic delivery, paving the way for systemic applications of the therapy. A number of novel and sophisticated technologies / platforms have been / are being developed to overcome the challenges associated with RNAi therapeutics, as well as further enhance their specificity and stability. Further, considering their high knockdown efficiency, high target specificity and extended silencing ability, RNAi has now been established as a powerful tool for gene silencing. It is worth highlighting, RNAi has potential applications in treating several viral infections and tumors in addition to its ability in gene function determination. However, despite the numerous benefits offered by RNAi therapies, developers often face concerns related to the stability and targeted delivery of their candidates. In order to mitigate the aforementioned challenges, several players have developed novel technologies and delivery systems to ensure target specific delivery of these molecules.
To request a sample copy / brochure of this report, please visit
https://www.rootsanalysis.com/....reports/278/request-
With four approved drugs and several therapy candidates being evaluated in late stages of clinical development, the RNAi therapeutics domain presents a significant opportunity for biopharmaceutical developers. In addition, a number of RNAi therapeutics are being developed to target rare and genetic clinical conditions, such as Alpha 1-Antitrypsin Disease, Alport Syndrome, Amyloidosis and Muscular Dystrophy (Facioscapulohumeral Muscular Dystrophy and Oculopharyngeal Muscular Dystrophy). It is also worth highlighting that over 2,100 patents have been filed / granted, highlighting the continuous pace of ongoing innovation in this field. Given the high research activity and ongoing technology advancements, the RNAi therapeutics market is poised to grow at a steady pace in the foreseen future. An insightful technology competitiveness analysis, benchmarking RNAi technologies, based on supplier power (in terms of company size and years of experience) and key technology specifications. The analysis was designed to enable stakeholder companies to compare their existing capabilities within and beyond their respective peer groups and identify opportunities to achieve a competitive edge in the industry.
For additional details please visit https://www.rootsanalysis.com/....reports/view_documen or email sales@rootsanalysis.com
You may also be interested in the following titles:
1. Targeted protein degradation market, 2022-2035
2. Cell Therapy Manufacturing Market, 2021-2030
3. Single-Use Upstream Bioprocessing Technology / Equipment Market, 2022-2035
About Roots Analysis
Roots Analysis is one of the fastest growing market research companies, sharing fresh and independent perspectives in the bio-pharmaceutical industry. The in-depth research, analysis and insights are driven by an experienced leadership team which has gained many years of significant experience in this sector. If you’d like help with your growing business needs, get in touch at info@rootsanalysis.com
Contact:
Ben Johnson
+1 (415) 800 3415
+44 (122) 391 1091
Ben.johnson@rootsanalysis.com
Facebook - https://www.facebook.com/RootsAnalysis
LinkedIn - https://www.linkedin.com/compa....ny/roots-analysis/my
Twitter - https://twitter.com/RootsAnalysis
Medium - https://medium.com/@RootsAnalysis
Pinterest - https://in.pinterest.com/RootsanalysisPin/_saved/
Quora - https://rootsanalysisinsights.quora.com/

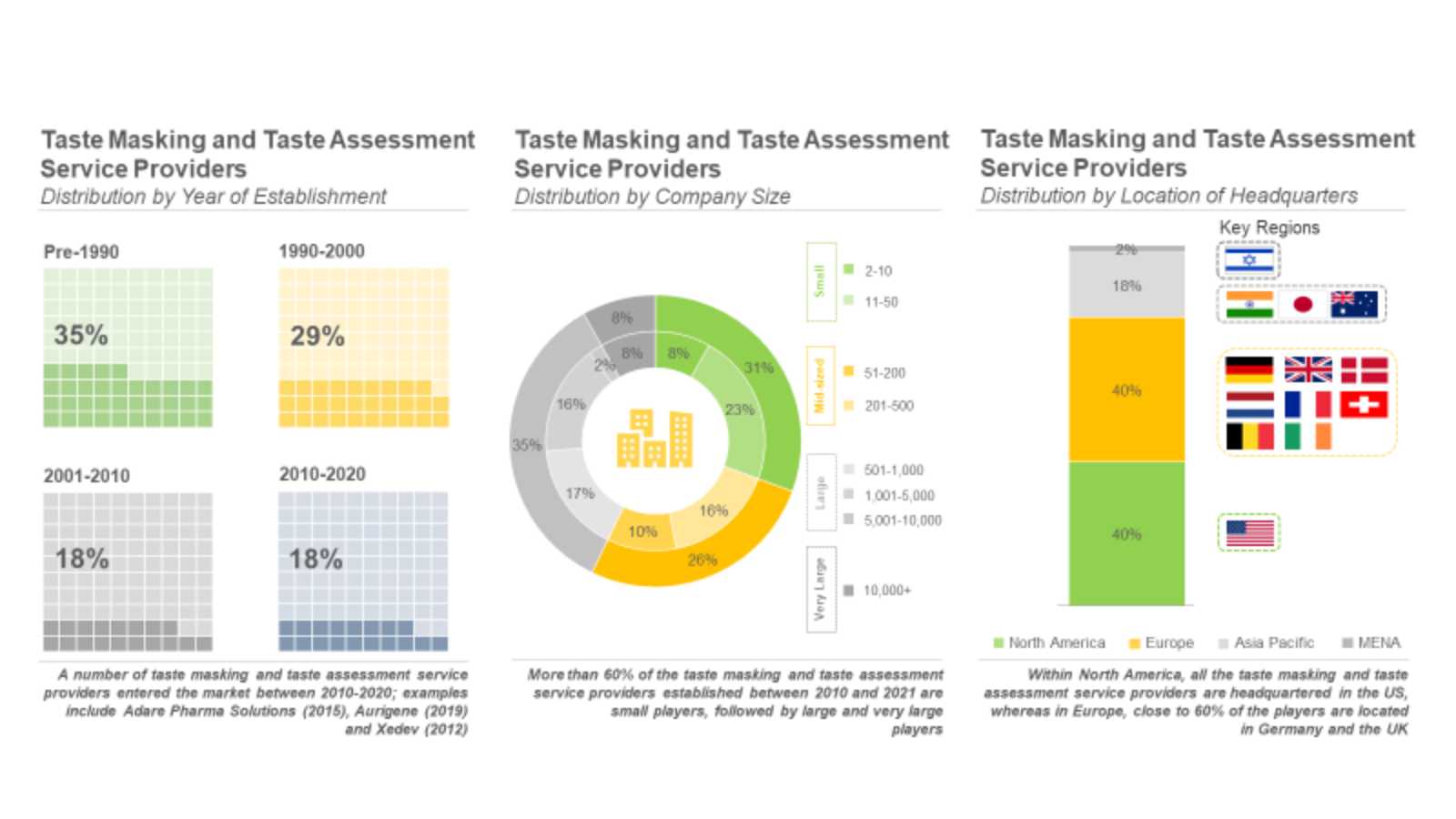
The taste masking market is anticipated to grow at a steady pace till 2035
Driven by the growing need to enhance palatability of oral drugs and drug adherence among pediatric and geriatric population, the demand for novel and advanced taste masking and taste assessment technologies is expected to rise in the coming years.
London
Roots Analysis has announced the addition of “Taste masking and Taste Assessment Services and Technologies Market, 2022-2035” report to its list of offerings.
The inherent expertise of CMOs and CDMOs in taste masking and taste assessment of bitter drug formulations, along with capabilities to identify globally accepted tastes, develop flavor matching placebo formulations (for testing) having compliance with stringent regulatory guidelines and good clinical practices (GCPs) and proprietary technologies offering significant cost-benefits, have rendered outsourcing as a crucial aspect of taste masked formulation development and production.
Key Market Insights
Presently, 50 companies claim to offer taste masking and taste assessment services for oral drug formulations
Majority (40%, each) of the service providers are based in North America and Europe, followed by companies headquartered in Asia-Pacific (18%). A large proportion (34%) of these companies are large players, followed by small (31%) and mid-sized firms (27%).
Close to 30 technology platforms have been developed for taste masking and taste assessment of oral drug formulations
Majority (68%) of the technology platforms are used for taste masking and taste assessment of solid oral formulations (tablets, capsules, granules and powder), followed by liquid (10%) oral formulations (suspensions, syrups and solutions).
Partnership activity in this field has grown significantly between 2018 and 2021
The maximum number of partnerships were established in 2021 indicating a recent rise in the interest of players engaged in the field of taste masking and taste assessment. It is worth highlighting that majority of the agreements were related to acquisition, representing 46% of the total number of partnerships signed. This is followed by agreements signed for manufacturing of oral drug formulation (13%).
More than 460 patents have been filed / granted for taste masking and taste assessment techniques and technologies, since 2017
Close to 60% of the patent applications have been filed by various industry and non-industry players in this domain post 2018. It is worth noting that, around 75% of the patents were filed / granted in the US, followed by European Patent Office (25%).
North America is anticipated to capture larger share of the market by 2035
The taste masking market is likely to be driven by technology platforms that employ coating techniques. Further, solid formulations are likely to hold greater market share.
To request a sample copy / brochure of this report, please visit
https://www.rootsanalysis.com/....reports/taste-maskin
The report features inputs from eminent industry stakeholders, who were very optimistic concerning the need of outsourcing of taste masking and taste assessment services in the coming decade. The report includes detailed transcripts of the discussions held with the following industry experts:
Phillipe Tschopp (Head of Business Development, Glatt Pharmaceutical Services)
David Tisi (Director of Technical Operations, Senopsys)
Brandon Keener (Business Development Associate, Adare Pharma Solutions)
For additional details, please visit https://www.rootsanalysis.com/....reports/taste-maskin or email sales@rootsanalysis.com
You may also be interested in the following titles:
1. Targeted protein degradation market, 2022-2035
2. Cell Therapy Manufacturing Market, 2021-2030
3. Single-Use Upstream Bioprocessing Technology / Equipment Market, 2022-2035
About Roots Analysis
Roots Analysis is one of the fastest growing market research companies, sharing fresh and independent perspectives in the bio-pharmaceutical industry. The in-depth research, analysis and insights are driven by an experienced leadership team which has gained many years of significant experience in this sector. If you’d like help with your growing business needs, get in touch at info@rootsanalysis.com
Contact:
Ben Johnson
+1 (415) 800 3415
+44 (122) 391 1091
Ben.johnson@rootsanalysis.com
Facebook - https://www.facebook.com/RootsAnalysis
LinkedIn - https://www.linkedin.com/compa....ny/roots-analysis/my
Twitter - https://twitter.com/RootsAnalysis
Medium - https://medium.com/@RootsAnalysis
Pinterest - https://in.pinterest.com/RootsanalysisPin/_saved/
Quora - https://rootsanalysisinsights.quora.com/
The human microbiome market is anticipated to grow at a CAGR of 24% till 2035
With the increasing concept of precision medicine, several scientists have demonstrated interest in the therapeutic manipulation of human microbiome (commonly gut bacteria) for the treatment of a wide range of disease indications.
Roots Analysis has announced the addition of “The Human Microbiome Market, (4th Edition) 2022-2035” report to its list of offerings.
The microbiome-based therapies pipeline features six drugs in phase III clinical development, over 200 candidates in other clinical and preclinical stages of development along with more than 60 diagnostics and screening / profiling tests that are commercialized for the detection of various diseases. However, the current microbiome market is driven by the fecal microbiota therapies approved by the FDA, particularly for the recurrent CDI and the commercialized diagnostic tests available to the patients. The human microbiome remained a largely unexplored area until 2007 when the Human Microbiome Project (HMP) was initiated. Given the role of microbiota in disease development and pathogenesis, the concept of microbiome-based therapeutics has generated significant enthusiasm within the medical science community, thereby, defining a new frontier in the field of medicine.
To request a sample copy / brochure of this report, please visit
https://www.rootsanalysis.com/....reports/281/request-
Key Market Insights
Over 230 drug candidates are currently being developed by more than 70 drug developers
Nearly 30% of the pipeline drugs are in clinical phase of development, while more than 150 drugs are in preclinical and discovery stages. Clinical stage drugs are primarily being developed for infectious diseases and digestive disorders, while candidates in preclinical and discovery stages are focused on oncological disorders.
Partnership activity in this domain has increased at a CAGR of 26%, between 2017 and 2021
Maximum number of partnerships were established in 2021, indicating a recent rise in the interest of players engaged in this domain. It is worth highlighting that majority of the deals were R&D agreements, representing over 35% of the total number of partnerships signed in the given time period.
More than USD 1 billion has been invested by both private and public investors, since 2017
Of the total amount invested, over USD 563 million was raised through venture capital financing, representing over 56% of the overall funding activity in this domain. Further, 36 instances of grants / awards were also reported, wherein players collectively raised more than USD 137 million.
Outsourcing has become an integral part of the microbiome and live biotherapeutics development process
Presently, over 25 service providers claim to offer a multitude of contract manufacturing services for microbiome and live biotherapeutic products. It is worth noting that close to 10 firms were established post 2010 and around 45% of the players are located in Europe.
Microbiome therapeutics are anticipated to capture more than 60% share of total microbiome market by 2035
As late-stage therapeutics will get approved by the FDA in the foreseen future, the microbiome therapeutics market is likely to grow at an annualized rate of 37% till 2035. It is worth mentioning that microbiome diagnostics is likely to capture 20% of the total microbiome market share by 2035.
For additional details, please visit https://www.rootsanalysis.com/....reports/view_documen or email sales@rootsanalysis.com
You may also be interested in the following titles:
1. Targeted protein degradation market, 2022-2035
2. Cell Therapy Manufacturing Market, 2021-2030
3. Single-Use Upstream Bioprocessing Technology / Equipment Market, 2022-2035
About Roots Analysis
Roots Analysis is one of the fastest growing market research companies, sharing fresh and independent perspectives in the bio-pharmaceutical industry. The in-depth research, analysis and insights are driven by an experienced leadership team which has gained many years of significant experience in this sector. If you’d like help with your growing business needs, get in touch at info@rootsanalysis.com
Contact:
Ben Johnson
+1 (415) 800 3415
+44 (122) 391 1091
Ben.johnson@rootsanalysis.com
Facebook - https://www.facebook.com/RootsAnalysis
LinkedIn - https://www.linkedin.com/compa....ny/roots-analysis/my
Twitter - https://twitter.com/RootsAnalysis
Medium - https://medium.com/@RootsAnalysis
Pinterest - https://in.pinterest.com/RootsanalysisPin/_saved/
Quora - https://rootsanalysisinsights.quora.com/
Oral Proteins and Peptides Market
Over the years, advances in recombinant DNA technology and ex vivo synthesis of biomolecules have led to the development and (in some cases) approval of several protein / peptide-based therapeutics.
Considering the therapeutic advantages associated with proteins and peptides, this field has witnessed significant activity; researchers are actively evaluating orally bioavailable interventions. However, owing to their inherently complex structure and comparatively low stability (in in vivo conditions), proteins / peptides are predominantly administered parenterally. Recent strides in drug delivery solutions have enabled scientists to successfully explore and exploit alternative routes of drug delivery, such as transdermal, intranasal, pulmonary and oral, for protein / peptide-based therapeutics. Of these, the oral route of delivery is considered to be the most patient-friendly. This has led several companies to invest in the development of orally administered biologics. The delivery of proteins and peptides utilizes the following routes of administration. Since the approval of the first protein / peptide-based therapy (recombinant human insulin) in 1982, there has been a substantial increase in the R&D initiatives focused on such products. Earlier, majority of the biologics were administered subcutaneously. However, with the technological advancements in delivery formulations, oral delivery of therapeutic interventions has gained significant traction, prompting stakeholders to leverage their expertise in the development of orally administrable proteins / peptides.
To request a sample copy / brochure of this report, please visit
https://www.rootsanalysis.com/....reports/192/request-
Although studies have reported that over XX oral drug delivery technologies have been designed so far, our research indicates that only XX% of the aforementioned technologies are currently being used to develop therapeutic proteins / peptides. Owing to the numerous advantages of oral drug delivery, including ease of administration, minimal pain and risk of drug reaction, and self-administration, we are led to believe that the oral proteins / peptide therapeutics market is anticipated to witness significant growth in the coming future.
For additional details, please visit https://www.rootsanalysis.com/....reports/view_documen or email sales@rootsanalysis.com
You may also be interested in the following titles:
1. Targeted protein degradation market, 2022-2035
2. Cell Therapy Manufacturing Market, 2021-2030
3. Single-Use Upstream Bioprocessing Technology / Equipment Market, 2022-2035
About Roots Analysis
Roots Analysis is one of the fastest growing market research companies, sharing fresh and independent perspectives in the bio-pharmaceutical industry. The in-depth research, analysis and insights are driven by an experienced leadership team which has gained many years of significant experience in this sector. If you’d like help with your growing business needs, get in touch at info@rootsanalysis.com
Contact:
Ben Johnson
+1 (415) 800 3415
+44 (122) 391 1091
Ben.johnson@rootsanalysis.com
Facebook - https://www.facebook.com/RootsAnalysis
LinkedIn - https://www.linkedin.com/compa....ny/roots-analysis/my
Twitter - https://twitter.com/RootsAnalysis
Medium - https://medium.com/@RootsAnalysis
Pinterest - https://in.pinterest.com/RootsanalysisPin/_saved/
Quora - https://rootsanalysisinsights.quora.com/

The needlestick safety injection devices market is anticipated to grow at a commendable pace by 2035
An exponential increase in the annual cost burden due to needlestick injuries, has fueled a corresponding rise in demand for safe and highly advanced safety devices to be developed by the stakeholders engaged in the healthcare industry to generate a stringent regulation for the prevention of needle-stick injuries.
Roots Analysis has announced the addition of “Needlestick Safety Injection Devices Market, 2022-2035” report to its list of offerings.
Given the inherent benefits of needlestick safety injection devices, number of developers have launched their proprietary devices for minimizing the risk of needlestick injuries. Moreover, stringent regulatory requirements have further prompted the stakeholders to improve product design and integrate better safety measures in their products, including needle shielding.
To request a sample copy / brochure of this report, please visit this https://www.rootsanalysis.com/....reports/needlestick-
Key Market Insights
Currently, around 120 needlestick safety injection devices are available in the market
More than 35% of these injection devices are needles having the ability to retract or shield, followed by syringes (30%). It is worth noting that more than 90% of the needlestick safety injection devices are non-reusable (intended for single use) thereby reducing the chances of blood borne pathogen infections.
Over 50 companies claim to develop needlestick safety injection devices, worldwide
Presently, the market is dominated by very small companies (33%) and small companies (25%). It is worth noting that majority (40%) of the firms engaged in this domain are based in Europe, followed by North America (39%).
Partnership activity within this field has grown significantly between 2020 and 2022
Maximum number of partnerships (31%) were established in 2020 indicating a recent rise in the interest of developers engaged in the development of needlestick safety injection devices. It is worth highlighting that majority of the deals were acquisitions.
Over USD 260 million has been invested by both private and public investors, since 2016
Companies involved in the development of needlestick safety injection devices have raised around USD 70 million through venture funding, which represents 25% of the total capital raised in the given time period. Overall, around 20 investors have actively financed various projects / initiatives in this domain.
Close to 20 global events were organized in the past couple of years in this industry
Majority of the events related to needlestick safety injection device were organized in North America (22%). It is worth highlighting that the main agenda of these events was to discuss role of needlestick safety injection devices to prevent the needlestick injuries.
More than 140 patents have been filed / granted related to needlestick safety injection devices, since 2018
Of these, over 60 patents were filed / granted in 2022 (till April). Industry players that have filed maximum number of patents related to needlestick safety injection devices include (in decreasing order of number of patents filed) BD, West Pharmaceutical Services, B. Braun, Retractable Technologies and Safety Syringes.
For additional details, please visit https://www.rootsanalysis.com/....reports/needlestick- or email sales@rootsanalysis.com
You may also be interested in the following titles:
1. Targeted protein degradation market, 2022-2035
2. Cell Therapy Manufacturing Market, 2021-2030
3. Single-Use Upstream Bioprocessing Technology / Equipment Market, 2022-2035
About Roots Analysis
Roots Analysis is one of the fastest growing market research companies, sharing fresh and independent perspectives in the bio-pharmaceutical industry. The in-depth research, analysis and insights are driven by an experienced leadership team which has gained many years of significant experience in this sector. If you’d like help with your growing business needs, get in touch at info@rootsanalysis.com
Contact:
Ben Johnson
+1 (415) 800 3415
+44 (122) 391 1091
Ben.johnson@rootsanalysis.com
Facebook - https://www.facebook.com/RootsAnalysis
LinkedIn - https://www.linkedin.com/compa....ny/roots-analysis/my
Twitter - https://twitter.com/RootsAnalysis
Medium - https://medium.com/@RootsAnalysis
Pinterest - https://in.pinterest.com/RootsanalysisPin/_saved/
Quora - https://rootsanalysisinsights.quora.com/
- 64 posts
-
- Male